

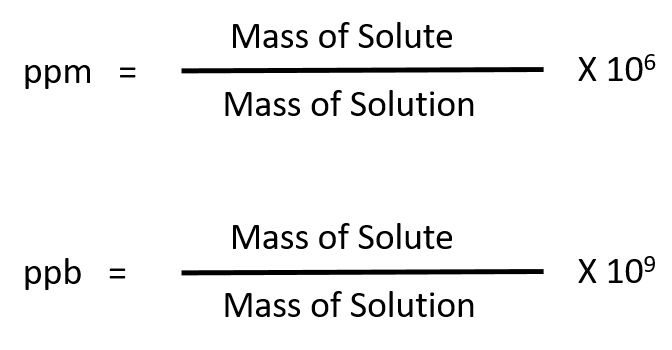
The mole fraction of solute i is written as X i. Mole Fraction: The mole fraction of a single solute in a solution is simply the number of moles of that solute divided by the total moles of all the solutes/solvents. The weight percent is designated by Wt% and sometimes w/w%. This can be in grams or kilograms as long as the units of both are expressed in the same manner. Weight Percent(or Mass Percent): The weight percent of a solution is calculated by taking the mass of a single solute and dividing it by the total mass of the solution. Molality is designated by a lower case "m". Molality: The molality of a solution is calculated by taking the moles of solute and dividing by the kilograms of solvent. Molarity: The molarity of a solution is calculated by taking the moles of solute and dividing by the liters of solution. But don't become too reliant on it since it will not be available during exams. I have added a conversion calculator at the bottom of this page to help you check your homework etc. You should know the meaning of each of these terms and more importantly how to convert from one to the other. There are Five main ways we describe the concentration of solutions: 1) Molarity 2) Molality 3) Weight Percent 4) Mole Fraction and 5) Parts Per Million or Billion.

The problems that might occur here stem from how much of this language you actually remember and whether or not you can apply what you remember to new problems. Fortunately, for discussing solutions, a great deal of the language was already covered in CHM1045. Whenever we begin to discuss a new subject we have to learn the language that accompanies it.


 0 kommentar(er)
0 kommentar(er)
